Fibrodysplasia ossificans progressiva (FOP) is an ultra-rare genetic disorder of extraskeletal endochondral bone formation. Heterozygous missense gain-of-function mutations in the activin receptor A type I (ACVR1) gene are present in all individuals with sporadic or familial FOP. ACVR1R206H mutations confer increased sensitivity of this bone morphogenetic protein (BMP) family receptor to activation by BMP ligands and alter responsiveness to normally antagonistic Activin ligands, especially Activin A.1 In patients with FOP, flares of heterotopic ossification (HO) are associated with accidental trauma or other inflammatory insults. This clinical observation has triggered intense investigation into the current model that tissue-resident immune cells participate in HO lesion initiation.2 Recently, the FDA approved palovarotene, a retinoic acid receptor gamma agonist that works in part by reducing Activin A expression in immune cells,3 as an agent that reduces the volume of new HO lesions in patients with FOP.4 Despite these advances, major questions remain regarding interrelationships between inflammation and ectopic ossification in FOP.
Although many monogenic Mendelian disorders exhibit complete penetrance, rare and common genetic variants can modify disease penetrance in the setting of other classic monogenic disorders including Huntington’s disease,5 cystic fibrosis,6 maturity onset diabetes of the young,7 and others. Identifying disease-modifying genes can provide prognostic information and suggest novel treatments. Applying systematic approaches to assess the contribution of genetic variants to FOP disease penetrance may be challenging given the low incidence of FOP. Furthermore, until now, the natural history of known FOP cases has suggested that patients bearing the ACVR1R206H mutation display a uniformly tragic course associated with immobilization by age 30 and early death due mainly to pulmonary complications.
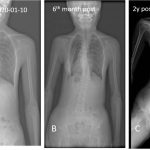
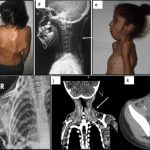
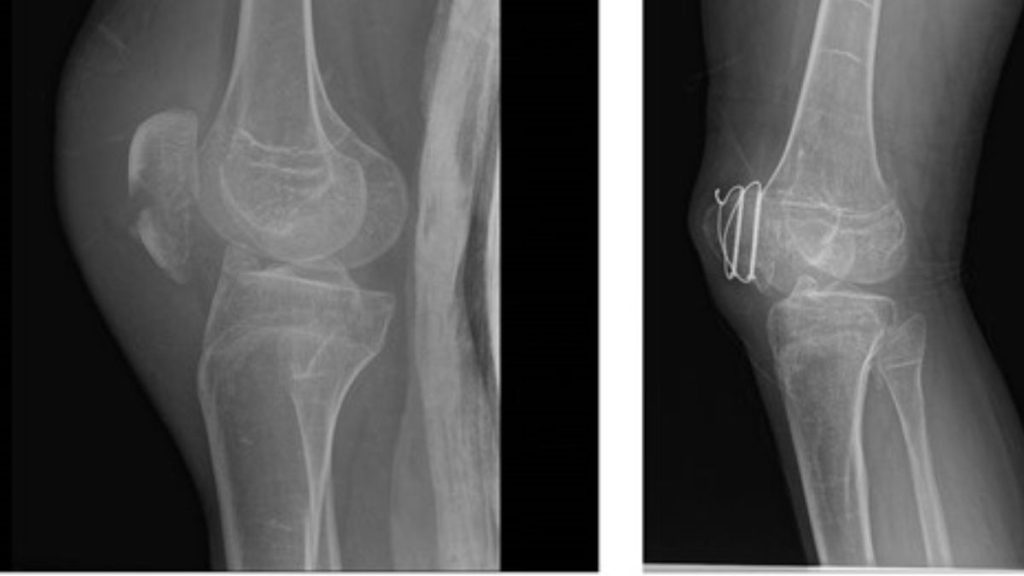
In this light, the current study by Lounev et al.8 is particularly exciting as a remarkable example of bedside-to-bench research. The authors reported the case of a male patient who first presented at age 20 with HO following surgical resection of a neck nodule. Notably, the patient was noted to lack interphalangeal joints in his thumbs and great toes, skeletal development anomalies seen in all patients with FOP, prompting genetic testing which confirmed a de novo ACVR1R206H mutation. A comprehensive examination for HO at that time revealed only small and asymptomatic disease burden, which remained relatively stable over subsequent years of observation, leading study investigators to note that this man has the “mildest form of classic FOP observed to date.”
An important clue into the etiology of this patient’s remarkable FOP resilience came from the analysis of a panel of circulating inflammatory biomarkers from 40 FOP patients vs matched controls. In this analysis, matrix metalloproteinase 9 (MMP-9) was identified as a circulating protein with very low levels and activity in this patient vs others studied. Whole exome sequencing identified that this patient is a compound heterozygote for A20V and D165N MMP-9 variants, and the D165N variant was predicted to reduce zinc binding and thus MMP-9 enzymatic function.
Prompted by these clinical and genetic observations, the authors turned to mice to study the role of MMP-9 in FOP pathogenesis. Remarkably, genetic ablation, even in the heterozygous state, of MMP-9 completely blocked HO lesion development in a FOP knockin mouse model, and inhibition of MMP-9 with either minocycline (an antibiotic that inhibits MMP-9) or a neutralizing MMP-9 antibody reduced HO lesions. At the mechanistic level, macrophages in the early inflammatory stage of FOP express high amounts of both MMP-9 and Activin A. Macrophage cell lines bearing the MMP-9 mutations seen in this patient showed reduced MMP-9 activity and reduced Activin A secretion. Finally, Activin A levels were measured in control and MMP-9-deficient FOP lesions. Despite the lack of HO formation, MMP-9 deficiency was associated with increased tissue Activin A levels, a finding interpreted as increased Activin A tissue sequestration. In other words, MMP-9 mediated proteolytic degradation may be needed for Activin A to be released from the extracellular matrix to activate the mutant BMP receptor during the inflammatory stages of HO progression.
In sum, these findings indicate that careful clinical observation of a single patient can uncover major new mechanistic insights into FOP pathogenesis. MMP-9 now emerges as a candidate therapeutic target in FOP, and perhaps in other forms of HO. Although previous studies had suggested that MMP-9, a major heparan sulfate degrading enzyme, is involved in normal endochondral bone formation9 and is expressed at sites of BMP2-induced HO,10 description of the critical role for MMP-9 in FOP pathogenesis in this study represents a major advance in the field. Pharmacologic MMP-9 inhibition may be a useful strategy to prevent lesion growth in MMP-9 “wild type” FOP patients. Whether tetracycline-based antibiotics, which can block MMP-9 activity at multiple levels, might affect HO in FOP patients thus emerges as a readily testable therapeutic hypothesis. Since such antibiotics likely have pleiotropic effects, complementary development of more specific and potent MMP-9 inhibitors also represents an exciting possibility.
Despite these advances, exciting questions for future research remain regarding the authors’ model. First, exhaustive scouring of genomic databases and careful scrutiny of patient phenotypic databases for great toe deformities will be needed to identify additional “resilient” ACVR1R206H mutation-bearing patients, and, therefore, additional genetic FOP modifiers. Second, further study is needed to unravel the precise relationship between MMP-9 and bioactive Activin A levels in early HO pathogenesis in FOP and other more common causes of HO. The finding that MMP-9 mutations reduce Activin A secretion by cultured macrophages yet increase “free” Activin A levels during HO progression suggests a complex relationship between these 2 critical HO-driving factors. In light of ongoing studies examining Activin A-blocking antibodies in FOP,11 understanding more details about how MMP-9 regulates the bioavailability of Activin A and other matrix-bound BMP-family ligands emerges as a major research priority. Finally, this work beautifully illustrates the concept that genetic modifiers exert an important influence on the natural history of “monogenic” skeletal diseases. Thus, this work underscores the critical importance of asking simple questions informed from our patients during “bedside-to-bench” research to gain new insights in rare bone diseases.
Source: Academic