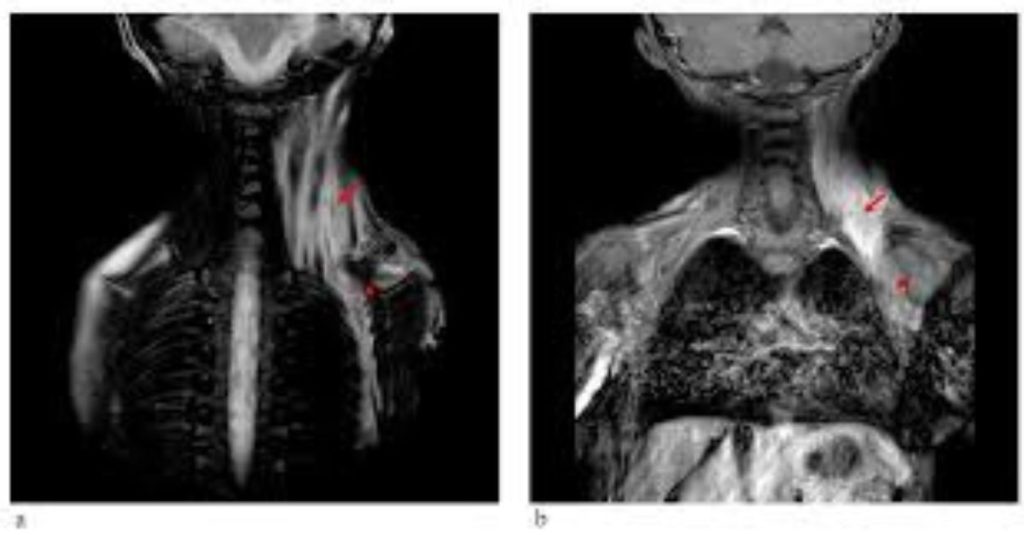
Purpose of the study Fibrodysplasia ossificans progressiva (FOP) is a rare autosomal dominant condition that leads to significant disability and morbidity, characterised by the formation of heterotopic hard tissues within connective tissues. The condition has an incidence of approximately one per two million people worldwide. There is no known single effective treatment available for FOP. […]
Currently browsing: Uncategorized
35 Years Later, FOP Connection Continues Bringing the Community Together
From a readership of 11 in 1988 to more than 2,000 in 2023, the IFOPA newsletter has evolved to inform and connect friends across the world Together with her sister-in-law, Anne, and fellow IFOPA founding member, Nancy Sando, Jeannie Peeper published the first edition of the FOP Connection in 1988. Back then, it looked quite a bit […]
‘This Assistance Has Been Life-Changing’
L.I.F.E. Award Grant makes it easier for professional with FOP to work from home Kathleen Degenhardt works from home. She also has FOP, which makes it difficult for her to perform some tasks. She learned about the Harold & Elaine Kaplan Quality of L.I.F.E. Award program while reading the IFOPA monthly newsletter and applied. The program provides […]
What’s New with In Pursuit of a Cure 2023?
Connect with FOP researchers, meet new families and more! You’ll notice a few things are brand new for this year’s In Pursuit of a Cure campaign. But the most important part of the campaign is the same — you! Read on for a quick update on some exciting changes and what they mean to you in the weeks ahead… We’ve supercharged In […]
Clementia Granted Rare Pediatric Disease Designation by FDA for Palovarotene for Fibrodysplasia Ossificans Progressiva
MONTREAL, Feb. 11, 2019 (GLOBE NEWSWIRE) — Clementia Pharmaceuticals Inc. (Nasdaq: CMTA), a clinical-stage biopharmaceutical company innovating treatments for people with ultra-rare bone disorders and other diseases, today announced that the U.S. Food and Drug Administration (FDA) has granted Rare Pediatric Disease designation to palovarotene for the treatment of fibrodysplasia ossificans progressiva (FOP). Palovarotene, an […]
Clementia Announces Plan to Submit a New Drug Application for Palovarotene for the Treatment of FOP Based on Positive Phase 2 Results
NDA Submission Planned for the Second Half of 2019 MONTREAL, Oct. 23, 2018 (GLOBE NEWSWIRE) — Clementia Pharmaceuticals Inc. (Nasdaq: CMTA), a clinical-stage biopharmaceutical company innovating treatments for people with ultra-rare bone disorders and other diseases, today announced that it plans to submit a New Drug Application (NDA) for palovarotene to the U.S. Food and […]
Blueprint Medicines Presents Foundational Preclinical Data Supporting the Development of BLU-782, a Highly Selective ALK2 Inhibitor, for the Treatment of Patients with Fibrodysplasia Ossificans Progressiva
Blueprint Medicines Corporation, a leader in discovering and developing targeted kinase medicines for patients with genomically defined diseases, today announced preclinical proof-of-concept data for BLU-782, an investigational oral precision therapy specifically designed to target mutant activin-like kinase 2, the underlying cause of fibrodysplasia ossificans progressiva. Preclinical studies in a well-characterized, genetically accurate FOP model showed […]
Clementia Announces Updated Phase 2 Part B Data on Palovarotene for FOP
Clementia Pharmaceuticals Inc., a clinical-stage biopharmaceutical company innovating treatments for people with ultra-rare bone disorders and other diseases, today announced updated data from the open label extension (“Part B”) of its ongoing Phase 2 clinical trial of palovarotene in fibrodysplasia ossificans progressiva (FOP). “These updated data continue to support the potential for palovarotene in FOP, […]
Human skeletal stem cells identified
Human skeletal stem cells that become bone, cartilage, or stroma cells have been isolated from fetal and adult bones. This is the first time that skeletal stem cells, which had been observed in rodent models, have been identified in humans. The researchers were also able to derive the skeletal stem cells from human induced pluripotent […]
Regeneron’s LUMINA-1 Trial hosted by FOP Friends
Learn more about Regeneron’s LUMINA-1 clinical trial presented by Dr. Richard Keen from the Royal National Orthopaedic Hospital in England. This webinar is hosted by FOP Friends in the United Kingdom http://fopfriends.com/. Source: FOPnews
Search
Recent Posts
-
 Optimized Genome-Editing Method Opens The Door To More Effective Treatment Of Genetic Diseases
Optimized Genome-Editing Method Opens The Door To More Effective Treatment Of Genetic Diseases -
 Fibrodysplasia Ossificans Progressiva
Fibrodysplasia Ossificans Progressiva -
 Fibrodysplasia Ossificans Progressiva Pipeline Report, 2024 Updates : In-Depth Analysis Into the Clinical Trials, Latest FDA, EMA, and PMDA Approvals, Emerging Drugs, Growth Prospects and Companies
Fibrodysplasia Ossificans Progressiva Pipeline Report, 2024 Updates : In-Depth Analysis Into the Clinical Trials, Latest FDA, EMA, and PMDA Approvals, Emerging Drugs, Growth Prospects and Companies -
 Successful Preimplantation Genetic Testing for Fibrodysplasia Ossificans Progressiva: a Case Report
Successful Preimplantation Genetic Testing for Fibrodysplasia Ossificans Progressiva: a Case Report -
 Actionable Disease Insights from Bedside-to-Bench Investigation in Fibrodysplasia Ossificans Progressiva
Actionable Disease Insights from Bedside-to-Bench Investigation in Fibrodysplasia Ossificans Progressiva

![]Life-Changing](https://foptrust.org/wp-content/uploads/2023/11/Untitled-design.png)





