The Fibrodysplasia Ossificans Progressiva Pipeline report embraces in-depth commercial and clinical assessment of the pipeline products from the pre-clinical developmental phase to the marketed phase. The report also covers a detailed description of the drug, including the mechanism of action of the drug, clinical studies, NDA approvals (if any), and product development activities comprising the […]
All posts tagged: Fibrodysplasia Ossificans Progressiva
Successful Preimplantation Genetic Testing for Fibrodysplasia Ossificans Progressiva: a Case Report
Purpose of the study Fibrodysplasia ossificans progressiva (FOP) is a rare autosomal dominant condition that leads to significant disability and morbidity, characterised by the formation of heterotopic hard tissues within connective tissues. The condition has an incidence of approximately one per two million people worldwide. There is no known single effective treatment available for FOP. […]
Fibrodysplasia Ossificans Progressiva: A Rare Disease Due to Unawareness, Case Report and Literature Review
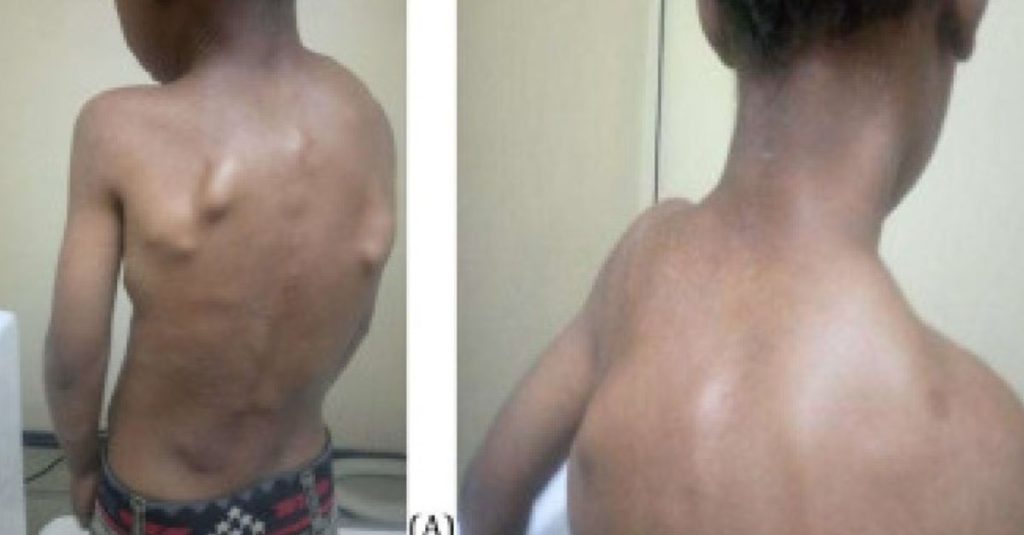
Abstract Introduction and importance Fibrodysplasia Ossificans Progressiva is an ultra-rare genetic disorder of progressive soft tissue ossification. Due to unawareness and poor clinical suspicion, the rate of misdiagnosis, delay in diagnosis, and unnecessary diagnostic procedures leading to permanent injury and lifelong disability is common. Here we report this rare genetic disorder in a six years […]
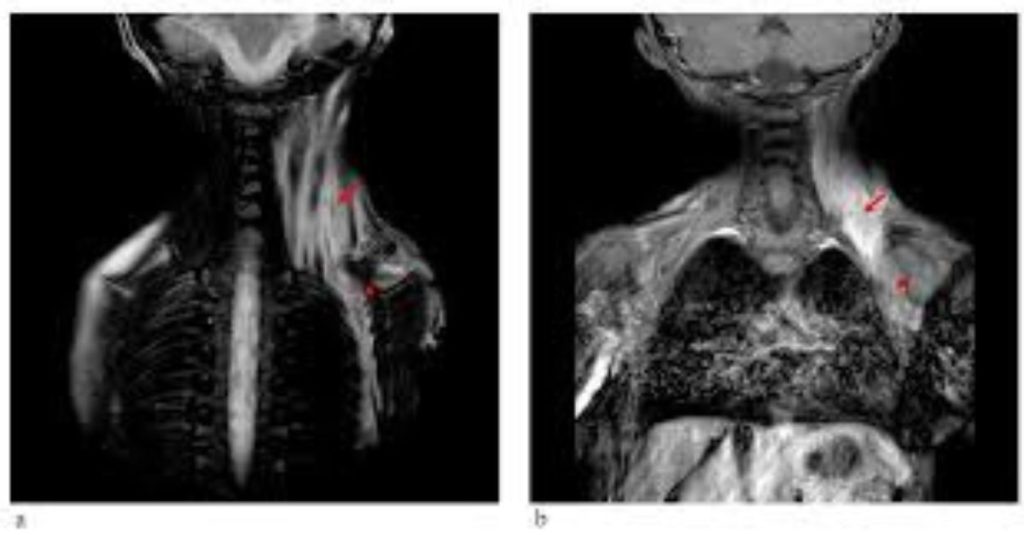
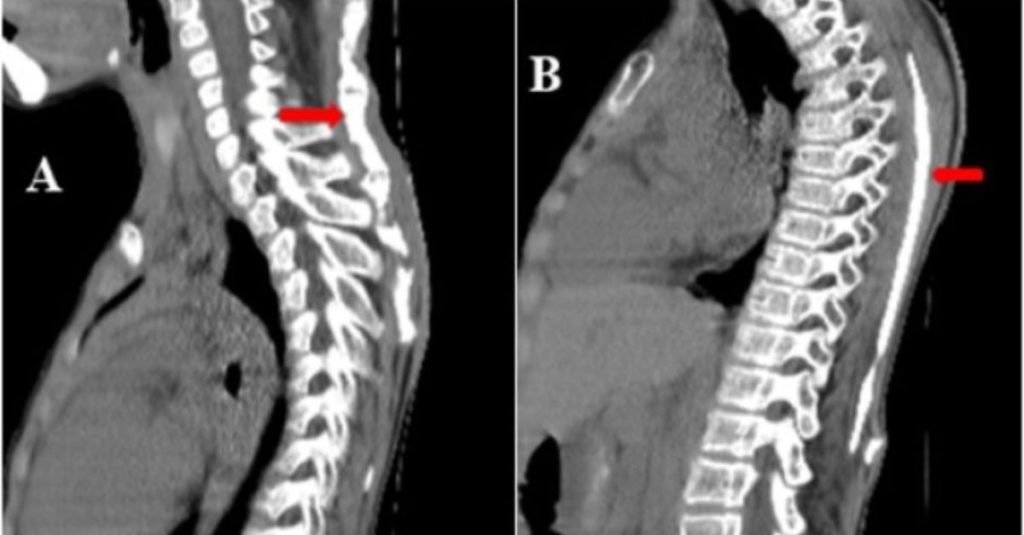
Fibrodysplasia ossificans progressiva: Two case reports
Introduction Fibrodysplasia ossificans progressiva (FOP) is a rare, progressively debilitating disorder first described in 1692 by Guy Patin [1]. This disease is characterized by progressive heterotopic ossification that forms normal bone in characteristic extraskeletal sites and congenital malformations of the great toes. Progressive episodes of heterotopic ossification occur in characteristic pattern first at the dorsal, axial, […]
FDA Approves First Treatment for Fibrodysplasia Ossificans Progressiva
Action The U.S. Food and Drug Administration (FDA) has approved Sohonos (palovarotene) capsules for reduction in the volume of new heterotopic ossification (extra-skeletal bone formation) in adults and children aged 8 years and older for females, and 10 years and older for males with fibrodysplasia ossificans progressiva. Sohonos is the first drug approved for patients with fibrodysplasia […]
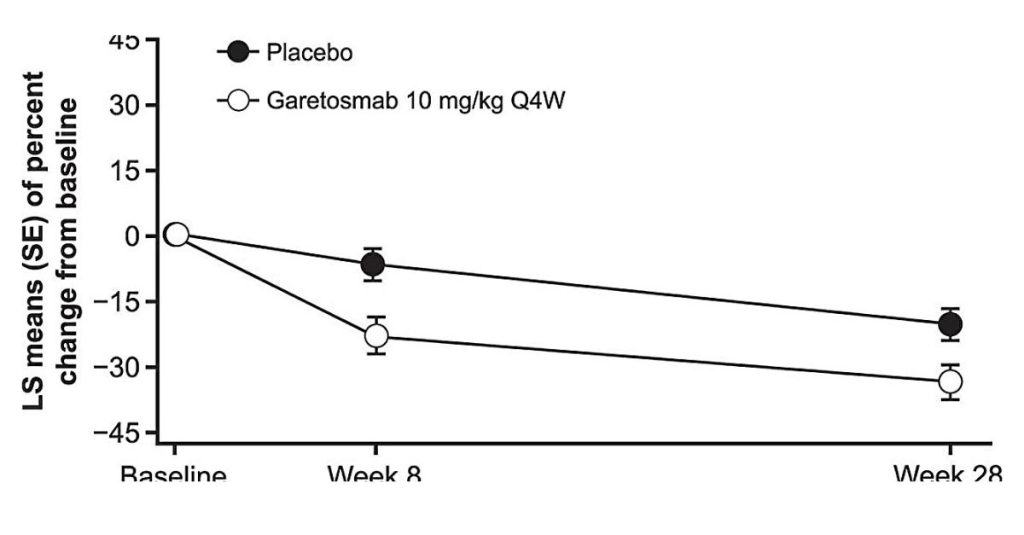
Drug Trial Shows Reduced Abnormal Bone Formation in Those with Fibrodysplasia Ossificans Progressiva
A multisite, international phase 2 trial evaluating the investigational drug garetosmab has shown that it reduced soft-tissue flare-ups significantly and prevented new areas of abnormal bone formation in patients with fibrodysplasia ossificans progressiva (FOP). Kathryn Dahir, MD, professor of Medicine in the Division of Endocrinology and Diabetes, served as the principal investigator at Vanderbilt University […]
Search
Recent Posts
-
 Optimized Genome-Editing Method Opens The Door To More Effective Treatment Of Genetic Diseases
Optimized Genome-Editing Method Opens The Door To More Effective Treatment Of Genetic Diseases -
 Fibrodysplasia Ossificans Progressiva
Fibrodysplasia Ossificans Progressiva -
 Fibrodysplasia Ossificans Progressiva Pipeline Report, 2024 Updates : In-Depth Analysis Into the Clinical Trials, Latest FDA, EMA, and PMDA Approvals, Emerging Drugs, Growth Prospects and Companies
Fibrodysplasia Ossificans Progressiva Pipeline Report, 2024 Updates : In-Depth Analysis Into the Clinical Trials, Latest FDA, EMA, and PMDA Approvals, Emerging Drugs, Growth Prospects and Companies -
 Successful Preimplantation Genetic Testing for Fibrodysplasia Ossificans Progressiva: a Case Report
Successful Preimplantation Genetic Testing for Fibrodysplasia Ossificans Progressiva: a Case Report -
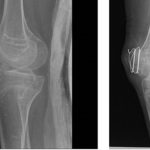 Actionable Disease Insights from Bedside-to-Bench Investigation in Fibrodysplasia Ossificans Progressiva
Actionable Disease Insights from Bedside-to-Bench Investigation in Fibrodysplasia Ossificans Progressiva