FOP is a rare genetic disease characterized by progressive and systemic heterotopic ossification (abnormal bone formation in soft tissues). Most FOP patients harbor mutations in the ACVR1 gene, encoding one of the type I BMP (Bone Morphogenic Protein) receptors. Through a previous collaboration with CiRA Associate Professor Makoto Ikeya, Toguchida’s team utilized iPS cells derived from FOP patients to identify a key molecule implicated in triggering heterotopic ossification. In that study, they found Activin A to erroneously transmit BMP signaling through mutant ACVR1, resulting in mTORC1 complex activation. This aberrant mTORC1 activation, in turn, promotes the chondrogenic differentiation of mesenchymal stem cells (MSCs) induced from FOP-iPSCs but not from mutation-rescued FOP-iPSCs. Based on these results, they initiated the clinical trials using a mTORC1 inhibitor, rapamycin. However, the molecular events downstream of activated mTORC1 remained unclear.
In the current study, the researchers first compared gene expression profiles of FOP-MSCs and mutation-rescued FOP-MSCs during chondrogenesis and revealed an enrichment of OXPHOS-associated genes in FOP-MSCs. Consistent with this, they uncovered the upregulation of TCA cycle metabolites and increased ATP production during chondrogenesis, respectively, through mass spectrometry and oxygen consumption rate measurements. Remarkably, treatment with IACS-010759 (IACS), an inhibitor of the mitochondrial respiratory complex I, significantly suppressed the chondrogenic differentiation of FOP-MSCs. These findings highlight the pivotal role of OXPHOS in Activin A-driven chondrogenesis of FOP-MSCs.
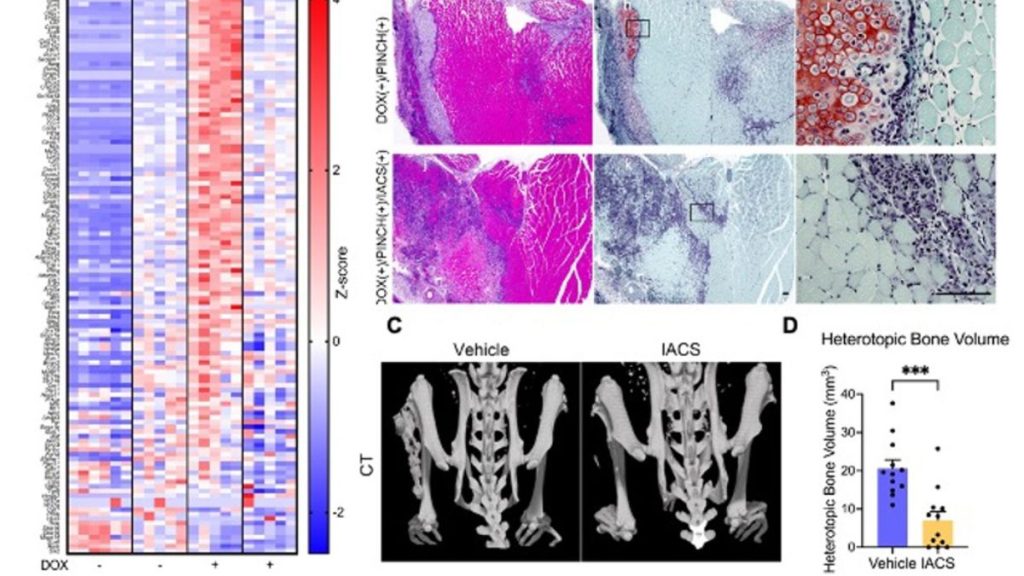
To examine the pathogenic contribution of OXPHOS in vivo, the researchers induced heterotopic ossification by pinch injury in FOP model mice carrying the mutated human ACVR1 gene. Consistent with in vitro findings, chondrogenic differentiation in injured tissues was inhibited by IACS treatment, resulting in the suppression of heterotopic ossification. Fibro-adipogenic progenitors (FAPs), identified as PDGFRα-positive cells in skeletal muscles possessing fibrogenic and adipogenic properties, are considered the pathogenic cells responsible for heterotopic ossification in FOP. Notably, the research team observed injury to trigger the upregulation of FAP-associated genes, which was attenuated by IACS treatment. Moreover, histological analysis revealed the injury-induced proliferation of PDGFRα-positive cells, which were also positive for TOMM20, a marker for mitochondrial biogenesis. Remarkably, treatment with IACS led to the disappearance of these PDGFRα and TOMM20 double-positive cells in injured tissues.
These findings indicate that IACS impedes chondrogenesis and heterotopic ossification by inhibiting OXPHOS activation in FAPs, thus advancing our understanding of the molecular mechanisms underlying heterotopic ossification in FOP as well as providing a novel therapeutic avenue for treatment.









