Introduction
Fibrodysplasia ossificans progressiva (FOP) is an ultra-rare genetic disease with an estimated prevalence of 1:1.2–1.5 million [1]. It is among the most catastrophic forms of heterotopic ossification (HO) [2] because of the progressive, irreversible accumulation of heterotopic bone and subsequent loss of mobility and independence. FOP is caused by activating mutations (mostly, the highly recurrent c.617G>A/p.R206H missense gain-of-function mutation) in the ACVR1/ALK2 gene, that encodes the type 1 activin A receptor and leads to aberrant activation of the bone morphogenetic protein pathway and new neo-ligand activity of the activin A receptor [3].
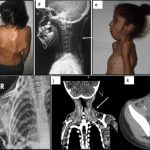
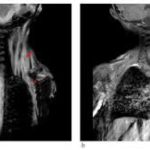
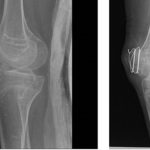
Clinically, FOP is characterized by severe cumulative heterotopic bone formation, but bilateral deformities of the great toe can be seen at birth in about 80% of patients [4]. Additional skeletal deformities are known [5]. Patients often experience painful, indurated soft tissue swellings and masses that develop in response to even mild trauma and often progress to HO masses [2]. These flare-ups are occasionally associated with elevated inflammatory markers and sometimes respond to anti-inflammatory agents [6–9]. Flare-ups often begin during the first decade of life. By the third decade, most FOP patients are wheelchair-bound [2]. A major cause of morbidity is extensive joint ankylosis. This includes the temporomandibular joints, with nutritional compromise; the intercostal joints and rib cage, with thoracic insufficiency syndrome and restricted respiratory function; and limb joints, with loss of ambulation [2]. HO at non-joint sites like the buttocks and back also prevent some patients from sitting comfortably or lead to pressure ulcers. Average life expectancy is about 50 years [2, 10].
Currently, only limited clinically effective disease-modifying treatments for FOP are available. Standard therapies include anti-inflammatory agents, such as anti-leukotrienes, glucocorticoids, non-steroidal anti-inflammatory drugs and mast-cell stabilizers. Palovarotene was approved by Health Canada (https://canjhealthtechnol.ca/index.php/cjht/article/view/SR0761/1400) and by the United States Food and Drug Administration (https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-first-treatment-fibrodysplasia-ossificans-progressiva) to reduce HO in females ages 8 years and older and males 10 years and older, diagnosed with FOP. However, the prevention of flare-ups and new HO sites remains inadequately managed [4].
FOP HO flare-ups occur via the bone morphogenetic protein pathway [8, 9] and share a striking resemblance to flares in well-characterized auto-inflammatory diseases, typified by innate immune cell activation, elicited by trivial triggers that usually do not cause a response in healthy individuals. The inflammatory response in these diseases is associated with overproduction of innate immune cytokines. IL-1β has been shown to have important roles in causing tissue damage, in some patients [11]. Accordingly, increasing evidence supports a major role for inflammation in HO Elevated pro-inflammatory cytokines and increased pro-inflammatory monocyte subsets have also been found in the blood of FOP patients [6]. Consistent with these observations, serum levels of TNF-α and IL-1β were significantly increased within 48 h post-injury, in a mouse model of trauma-induced HO.
There is an increased incidence of flare-ups in younger FOP patients, mostly below the age of 15 years [2], which indicates higher disease activity levels during childhood. The progressive, cumulative nature of HO in FOP indicates that interventions in early childhood are critical to reduce the lifelong, cumulative consequences of HO in FOP.
Therapies targeting IL-1, such as anakinra (IL-1 receptor antagonist), or canakinumab (anti-IL-1β monoclonal antibody) have extensive paediatric experience [11]. We previously reported that treatment with anakinra or canakinumab significantly reduced FOP flare-ups in a patient. This patient also demonstrated increased responsiveness to glucocorticoids used to manage flare-ups [15].
Here, we report our long-term experience with IL-1 inhibitors for preventing FOP flare-ups and managing FOP in 4 patients from 2 medical centres.
Methods
Patients were diagnosed based on typical clinical features of FOP and carried the c.617G>A/p.R206H mutation. All had severe, active disease, with paroxysmal HO flares, despite standard-of-care therapy. Flare-up activity was based on patients’ and parents’ reports and diaries, according to symptoms, including pain, swelling, erythema, induration and acute loss of motion. In addition, these were verified by the physicians through physical examinations in all cases and imaging tests, when performed. These were all documented in the medical records, with times in relation to the reports from the patients and their families. Parents of patients treated with IL-1 inhibition therapy completed the Child Health Assessment Questionnaire (CHAQ) questionnaire to better understand their functional status, after being on IL-1 inhibition therapy for over 1 year. We focused on changes in the pain and general health visual analogue scales (VAS) sections.
Cytokine measurements
Blood samples were obtained during flare activity or routine follow-up while under biologic treatment. Plasma IL-1β levels were measured using the Human IL-1β/IL-1F2 Quantikine High Sensitivity ELISA Kit (HSLB00D, R&D Systems, Inc., Minneapolis, MN).
Statistics
Flare frequency was determined by the number of documented flare-ups over the treatment period and expressed as flare-ups/month. Flare frequency for the pre-IL-1 inhibitor, IL-1 inhibitor pauses and during IL-1 therapy phases, were combined and averaged. Statistical significance for anti-IL-1 treatment vs non-treatment phases was determined using a two-tailed paired t-test (Excel, Microsoft Corporation).
Role of the funding source
The funding sources had no role in the writing of the manuscript or in the decision to submit it for publication.
Case presentations
Three female and 1 male patient with FOP, diagnosed clinically (toe malformations, FOP-like indurated flares and generalized HO) and genetically (ACVR1R206H mutation) and higher than average flare activity [16], were treated with IL-1 inhibitors. IL-1 inhibition onset ranged from 23 months to 15 years (Table 1). All received chronic standard-of-care treatment [4], plus palovarotene [17] (patients 2 and 4), intermittent pamidronate infusions (patient 1), sirolimus (patient 2), imatinib [18] (patient 4) or tranexamic acid (patient 3). Nonetheless, all experienced persistent flare activity before IL-1 inhibitors were initiated.