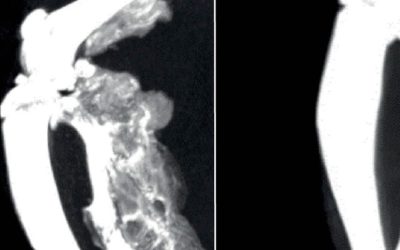
The participation of patients with a diagnosis of classic fibrodysplasia ossificans progressiva (FOP) who have previously undergone surgery is being sought for a study that involves the review of medical records.
We are asking for the participation of patients who have the diagnosis of FOP confirmed by sequencing, and have previously undergone surgical excision of HO for the purpose of restoring mobility to a joint.
Patients will have undergone computed tomography (CT) imaging studies of the surgically treated joint within 3 months before the surgery, within 2 weeks after the surgery, and at least one CT study between 6 months to 2 years following surgery, and are willing to make these scans available to the study staff. The patients will have been treated with conventional medical therapy for FOP, which may include corticosteroids, bisphosphonates, anti-histamine, leukotriene inhibitor, mast cell degranulation inhibitor, non-steroidal anti-inflammatory, and non-narcotic or narcotic analgesic agents.
To participate, patients and/or guardians will provide informed consent for this non-interventional study, and authorize release of medical records, genetic testing results, and associated CT scans through referring physicians and medical centers.
These data are being used to enable future clinical trials to permit surgical intervention in FOP.