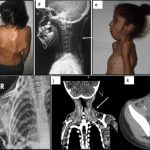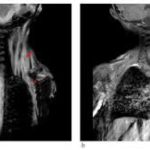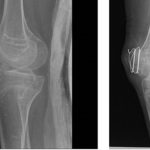
BLU-782 is effective in preventing heterotopic ossification after fibular osteotomy. Credit: Science Translational Medicine (2024). DOI: 10.1126/scitranslmed.abp8334
Several teams of medical researchers are currently testing new therapies for slowing or stopping the excess or runaway bone growth associated with fibrodysplasia ossificans progressive (FOP)—a rare bone disease.
The team behind one such effort, with members from Blueprint Medicines Corporation, Invicro and Alexion Pharmaceuticals, all in the Boston area, have published their findings in the journal Science Translational Medicine.
Until recently, it was not known what causes FOP—a disease so rare that only 8,000 people are currently under treatment for it. Then, in 2006, it was found to be genetically based—all of those with the affliction have a mutation in their ALK2 gene. It is still not clear how the gene causes excess amounts of bone growth, but researchers suspect the involvement of messaging that incites overactivation of stem cells.
The only treatment for the condition, until recently, has been surgery to remove excess bone, which has often led to accelerated bone growth, making the problem worse. Because of that, FOP is considered to be a fatal disease, though it can take 50 years.
Recently, the drug palovarotene was approved by several countries for treatment of FOP, but it has issues. It cannot be taken by children because it stunts growth, and it is too expensive for most patients.
- Full view of ALK2 kinase domain. Credit: Science Translational Medicine (2024). DOI: 10.1126/scitranslmed.abp8334
- Close-up view of the ALK2 ATP binding site. Credit: Science Translational Medicine (2024). DOI: 10.1126/scitranslmed.abp8334
In their effort, the team in Boston developed a therapy called BLU-782—it works by binding to the signal proteins that are activated by the ALK2 mutation, preventing them from signaling. It showed promise in mouse models before the instigation of clinical trials.
Preliminary results have shown administration of limited amounts of their small-molecule therapy has led to reductions in edema and prevention of cartilage and heterotopic ossification in both muscle tissue and bone injury sites.
Work is also progressing by four other teams involving 13 companies actively looking to develop treatments FOP—three are also in clinical trials. Those three are looking to prevent signaling from ALK2 by striking at its source. It is not yet clear how well such efforts are performing.
One thing all the efforts have in common is their focus on stopping the progression of the disease—preventing it would involve genetic engineering to repair the faulty gene.
More information: Alison J. Davis et al, An ALK2 inhibitor, BLU-782, prevents heterotopic ossification in a mouse model of fibrodysplasia ossificans progressiva, Science Translational Medicine (2024). DOI: 10.1126/scitranslmed.abp8334
© 2024 Science X Network
Citation: Ongoing clinical trials offer hope for people born with genetic mutation behind fibrodysplasia ossificans (2024, May 30) retrieved 30 May 2024 from https://medicalxpress.com/news/2024-05-ongoing-clinical-trials-people-born.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.